Will electric car batteries improve

MIT Technology Review
Solid-state batteries can use a wide range of chemistries, but a leading candidate for commercialization uses lithium metal. Quantumscape, for one, is focused on that technology and raised hundreds of millions in funding before going public in 2020. The company has a deal with Volkswagen that could put its batteries in cars by 2025.
But completely reinventing batteries has proved difficult, and lithium-metal batteries have seen concerns about degradation over time, as well as manufacturing challenges. Quantumscape announced in late December it had delivered samples to automotive partners for testing, a significant milestone on the road to getting solid-state batteries into cars. Other solid-state-battery players, like Solid Power, are also working to build and test their batteries. But while they could reach major milestones this year as well, their batteries wont make it into vehicles on the road in 2023.
Solid-state batteries arent the only new technology to watch out for. Sodium-ion batteries also swerve sharply from lithium-ion chemistries common today. These batteries have a design similar to that of lithium-ion batteries, including a liquid electrolyte, but instead of relying on lithium, they use sodium as the main chemical ingredient. Chinese battery giant CATL reportedly plans to begin mass-producing them in 2023.
Sodium-ion batteries may not improve performance, but they could cut costs because they rely on cheaper, more widely available materials than lithium-ion chemistries do. But its not clear whether these batteries will be able to meet needs for EV range and charging time, which is why several companies going after the technology, like US-based Natron, are targeting less demanding applications to start, like stationary storage or micromobility devices such as e-bikes and scooters.
Today, the market for batteries aimed at stationary grid storage is smallabout one-tenth the size of the market for EV batteries, according to Yayoi Sekine, head of energy storage at energy research firm BloombergNEF. But demand for electricity storage is growing as more renewable power is installed, since major renewable power sources like wind and solar are variable, and batteries can help store energy for when its needed.
Lithium-ion batteries arent ideal for stationary storage, even though theyre commonly used for it today. While batteries for EVs are getting smaller, lighter, and faster, the primary goal for stationary storage is to cut costs. Size and weight dont matter as much for grid storage, which means different chemistries will likely win out.
One rising star in stationary storage is iron, and two players could see progress in the coming year. Form Energy is developing an iron-air battery that uses a water-based electrolyte and basically stores energy using reversible rusting. The company recently announced a $760 million manufacturing facility in Weirton, West Virginia, scheduled to begin construction in 2023. Another company, ESS, is building a different type of iron battery that employs similar chemistry; it has begun manufacturing at its headquarters in Wilsonville, Oregon.
Shifts within the standard
Lithium-ion batteries keep getting better and cheaper, but researchers are tweaking the technology further to eke out greater performance and lower costs.
Electric vehicle battery
Battery used to power the electric motors of a battery electric vehicle or hybrid electric vehicle
For the starting, lighting and ignition system battery of an automobile, see
Automotive battery.

An electric vehicle battery (EVB, also known as a traction battery) is a rechargeable battery used to power the electric motors of a battery electric vehicle (BEV) or hybrid electric vehicle (HEV).
Electric vehicle batteries differ from starting, lighting, and ignition (SLI) batteries, as they are typically lithium-ion batteries that are designed for high power-to-weight ratio and energy density. Smaller, lighter batteries are desirable because they reduce the weight of the vehicle and therefore improve its performance. Compared to liquid fuels, most current battery technologies have much lower specific energy, and this often impacts the maximum range of all-electric vehicles. Unlike earlier battery chemistries, notably nickelcadmium, lithium-ion batteries can be discharged and recharged daily and at any state of charge. Other types of rechargeable batteries used in electric vehicles include leadacid, nickelcadmium, nickelmetal hydride, and others.[1]
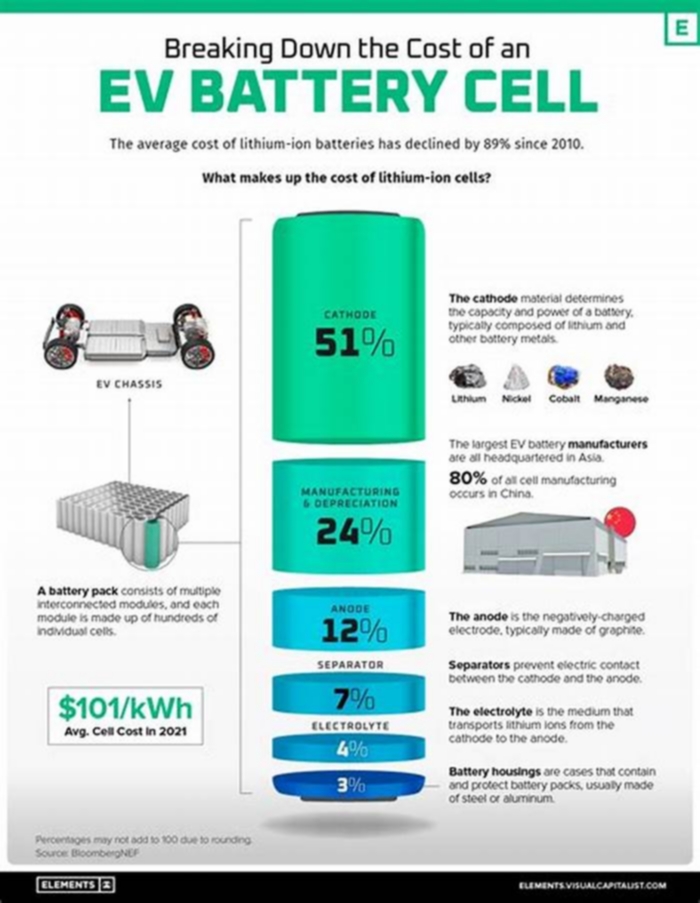
The battery makes up a significant portion of the cost and environmental impact of an electric vehicle. Growth in the industry has generated interest in securing ethical battery supply chains, which presents many challenges and has become an important geopolitical issue. As of December2019[update], the cost of electric vehicle batteries has fallen 87% since 2010 on a per kilowatt-hour basis.[2] As of 2018, vehicles with over 250mi (400km) of all-electric range, such as the Tesla Model S, are available.[3]
The price of electricity to run an electric vehicle is a small fraction of the cost of fuel for equivalent internal combustion engines, reflecting higher energy efficiency.[4]
Electric vehicle battery types[edit]
Lithium-ion[edit]
Lithium-ion (and the mechanistically similar lithium polymer) batteries, were initially developed and commercialized for use in laptops and consumer electronics. With their high energy density and long cycle life they have become the leading battery type for use in EVs. The first commercialized lithium-ion chemistry was a lithium cobalt oxide cathode and a graphite anode first demonstrated by N. Godshall in 1979, and by John Goodenough, and Akira Yoshino shortly thereafter.[5][6][7][8] The downside of traditional lithium-ion batteries include sensitivity to temperature, low temperature power performance, and performance degradation with age.[9] Due to the volatility of organic electrolytes, the presence of highly oxidized metal oxides, and the thermal instability of the anode SEI layer, traditional lithium-ion batteries pose a fire safety risk if punctured or charged improperly.[10] These early cells did not accept or supply charge when extremely cold, and so heaters can be necessary in some climates to warm them. The maturity of this technology is moderate. The Tesla Roadster (2008) and other cars produced by the company used a modified form of traditional lithium-ion "laptop battery" cells.
Recent EVs are using new variations on lithium-ion chemistry that sacrifice specific energy and specific power to provide fire resistance, environmental friendliness, rapid charging (as quickly as a few minutes), and longer lifespans. These variants (phosphates, titanates, spinels, etc.) have been shown to have a much longer lifetime, with A123 types using lithium iron phosphate lasting at least more than 10 years and more than 7000 charge/discharge cycles,[11] and LG Chem expecting their lithiummanganese spinel batteries to last up to 40years.[citation needed]
Much work is being done on lithium-ion batteries in the lab.[12] Lithium vanadium oxide has already made its way into the Subaru prototype G4e, doubling energy density.[13] Silicon nanowires,[14][15] silicon nanoparticles,[16] and tin nanoparticles[17][18] promise several times the energy density[clarification needed] in the anode, while composite[19][20] and superlattice[21] cathodes also promise significant density improvements.
New data has shown that exposure to heat and the use of fast charging promote the degradation of Li-ion batteries more than age and actual use, and that the average electric vehicle battery will retain 90% of its initial capacity after six years and six months of service. For example, the battery in a Nissan Leaf will degrade twice as fast as the battery in a Tesla, because the Leaf does not have an active cooling system for its battery.[22]


Lithium iron phosphate[edit]
LFP is a type of Li ion battery.[23] Although shorter range and lower charging performance they are cheaper and safer than NMC.[24]
Nickelmetal hydride[edit]

NiMH is also a type of Li ion battery.[23] Nickelmetal hydride batteries are now considered a relatively mature technology.[25] While less efficient (6070%) in charging and discharging than even leadacid, they have a specific energy of 3080Wh/kg, far higher than leadacid. When used properly, nickelmetal hydride batteries can have exceptionally long lives, as has been demonstrated in their use in hybrid cars and in the surviving first-generation NiMH Toyota RAV4EVs that still operate well after 100,000 miles (160,000km) and over a decade of service. Downsides include the poor efficiency, high self-discharge, very finicky charge cycles, and poor performance in cold weather.[citation needed]
GM Ovonic produced the NiMH battery used in the second generation EV-1, and Cobasys makes a nearly identical battery (ten 1.2V 85Ah NiMH cells in series in contrast with eleven cells for Ovonic battery). This worked very well in the EV-1.[26] Patent encumbrance has limited the use of these batteries in recent years.[citation needed]
Leadacid[edit]
Previously, most electric vehicles used leadacid batteries due to their mature technology, high availability, and low cost, with the notable exception of some early BEVs, such as the Detroit Electric which used a nickeliron battery. Leadacid batteries powered such early modern EVs as the original versions of the EV1. Flooded leadacid batteries are the oldest, cheapest, and, in the past, most common vehicle batteries available. There are two main types of leadacid batteries: automobile engine starter batteries, and deep-cycle batteries. Automobile engine starter batteries are designed to use a small percentage of their capacity to provide high charge rates to start the engine, while deep-cycle batteries are used to provide continuous electricity to run electric vehicles like forklifts or golf carts. Deep-cycle batteries are also used as the auxiliary batteries in recreational vehicles, but they require different, multi-stage charging.[27] No lead acid battery should be discharged below 50% of its capacity, as it shortens the battery's life.[27] Flooded batteries require inspection of electrolyte levels and occasional replacement of water, which gases away during the normal charging cycle.
The sodium nickel chloride or "Zebra" battery uses a molten sodium chloroaluminate (NaAlCl4) salt as the electrolyte. A relatively mature technology, the Zebra battery has a specific energy of 120Wh/kg. Since the battery must be heated for use, cold weather does not strongly affect its operation except for increasing heating costs. They have been used in several EVs[citation needed] such as the Modec commercial vehicle.[28][unreliable source?]Zebra batteries can last for a few thousand charge cycles and are nontoxic. The downsides to the Zebra battery include poor specific power (<300W/kg) and the requirement of having to heat the electrolyte to about 270C (518F), which wastes some energy, presents difficulties in long-term storage of charge, and is potentially a hazard.
Battery capacity[edit]
Capacities range from hundreds of watt-hours for ebikes to tens of thousands of watt-hours for ships.[29]
Supply chain[edit]
The electric vehicle supply chain comprises the mining and refining of raw materials and the manufacturing processes that produce lithium ion batteries and other components for electric vehicles. The lithium-ion battery supply chain is a major component of the overall EV supply chain, and the battery accounts for 3040% of the value of the vehicle.[30] Lithium, cobalt, graphite, nickel, and manganese are all critical minerals that are necessary for electric vehicle batteries.[31] There is rapidly growing demand for these materials because of growth in the electric vehicle market, which is driven largely by the proposed transition to renewable energy. Securing the supply chain for these materials is a major world economic issue.[32] Recycling and advancement in battery technology are proposed strategies to reduce demand for raw materials.Supply chain issues could create bottlenecks, increase costs of EVs and slow their uptake.[30][33]
The battery supply chain faces many challenges. Deposits of critical minerals are concentrated in a small number of countries, mostly in the
Global South. Mining these deposits presents dangers to nearby communities because of weak regulation, corruption, and
environmental degradation. These communities face
human rightsviolations,
environmental justiceissues, problems with child labour, and potentially generational legacies of contamination from mining activities. Manufacture of battery technology is largely dominated by China. However burning less
petroleum productsin vehicles can reduce the
environmental impact of the petroleum industrybecause, as of 2023
[update], most petroleum is used in vehicles.
[34]Lifecycle of lithium-based EV batteries[edit]

There are mainly four stages during the lifecycle of lithium-based EV batteries: the raw materials phase, the battery manufacturing, operation phase and the end-of-life management phase. As shown in the schematic of life cycle of EV batteries, during the first stage, the rare earth materials are extracted in different parts of the world. After they are refined by pre-processing factories, the battery manufacturing companies take over these materials and start to produce batteries and assemble them into packs. These battery packs are then sent to car manufacturing companies for EV integration. In the last stage, if no management is in place, valuable materials in the batteries could be potentially wasted. A good end-of-life management phase will try to close the loop. The used battery packs will either be reused as stationary storage or recycled depending on the battery state of health (SOH).[35]
The battery lifecycle is rather long and requires close cooperation between companies and countries. Currently, the raw materials phase and the battery manufacturing and operation phase are well established. The end-of-life management phase is struggled to grow, especially the recycling process mainly because of economics. For example, only 6% of lithium-ion batteries were collected for recycling in 20172018 in Australia.[36] However, closing the loop is extremely important. Not only because of a predicted tightened supply of nickel, cobalt and lithium in the future, also recycling EV batteries has the potential to maximize the environmental benefit. Xu et al. predicted that in the sustainable development scenario, lithium, cobalt and nickel will reach or surpass the amount of known reserves in the future if no recycling is in place.[37] Ciez and Whitacre found that by deploying battery recycling some green house gas (GHG) emission from mining could be avoided.[38]


To develop a deeper understanding of the lifecycle of EV batteries, it is important to analyze the emission associated with different phases. Using NMC cylindrical cells as an example, Ciez and Whitacre found that around 9kg CO2e kg battery1 is emitted during raw materials pre-processing and battery manufacturing under the US average electricity grid. The biggest part of the emission came from materials preparation accounting for more than 50% of the emissions. If NMC pouch cell is used, the total emission increases to almost 10kg CO2e kg battery1 while materials manufacturing still contributes to more than 50% of the emission.[38] During the end-of-life management phase, the refurbishing process adds little emission to the lifecycle emission. The recycling process, on the other hand, as suggested by Ciez and Whitacre emits a significant amount of GHG. As shown in the battery recycling emission plot a and c, the emission of the recycling process varies with the different recycling processes, different chemistry and different form factor. Thus, the net emission avoided compared to not recycling also varies with these factors. At a glance, as shown in the plot b and d, the direct recycling process is the most ideal process for recycling pouch cell batteries, while the hydrometallurgical process is most suitable for cylindrical type battery. However, with the error bars shown, the best approach cannot be picked with confidence. It is worth noting that for the lithium iron phosphates (LFP) chemistry, the net benefit is negative. Because LFP cells lacks cobalt and nickel which are expensive and energy intensive to produce, it is more energetically efficient to mine. In general, in addition to promoting the growth of a single sector, a more integrated effort should be in place to reduce the lifecycle emission of EV batteries. A finite total supply of rare earth material can apparently justify the need for recycling. But the environmental benefit of recycling needs closer scrutiny. Based on current recycling technology, the net benefit of recycling depends on the form factors, the chemistry and the recycling process chosen.
Manufacturing[edit]
There are mainly three stages during the manufacturing process of EV batteries: materials manufacturing, cell manufacturing and integration, as shown in Manufacturing process of EV batteries graph in grey, green and orange color respectively. This shown process does not include manufacturing of cell hardware, i.e. casings and current collectors. During the materials manufacturing process, the active material, conductivity additives, polymer binder and solvent are mixed first. After this, they are coated on the current collectors ready for the drying process. During this stage, the methods of making active materials depend on the electrode and the chemistry. For the cathode, two of the most popular chemistry are transition metal oxides, i.e. Lithium nickel manganese cobalt oxides (Li-NMC) and Lithium metal phosphates, i.e. Lithium iron phosphates (LFP). For the anode, the most popular chemistry now is graphite. However, recently there have been a lot of companies started to make Si mixed anode (Sila Nanotech, ProLogium) and Li metal anode(Cuberg, Solid Power). In general, for active materials production, there are three steps: materials preparation, materials processing and refinement. Schmuch et al. discussed materials manufacturing in greater details.[39]
In the cell manufacturing stage, the prepared electrode will be processed to the desired shape for packaging in a cylindrical, rectangular or pouch format. Then after filling the electrolytes and sealing the cells, the battery cells are cycled carefully to form SEI protecting the anode. Then, these batteries are assembled into packs ready for vehicle integration. Kwade et al. discuss the overall battery manufacturing process in greater detail.
Reusing and repurposing[edit]
When an EV battery pack degrades to 70% to 80% of its original capacity, it is defined to reach the end-of-life. One of the waste management methods is to reuse the pack. By repurposing the pack for stationary storage, more value can be extracted from the battery pack while reducing the per kWh lifecycle impact. However, enabling battery second-life is not easy. Several challenges are hindering the development of the battery refurbishing industry.
First, uneven and undesired battery degradation happens during EV operation. Each battery cell could degrade differently during operation. Currently, the state of health (SOH) information from a battery management system (BMS) can be extracted on a pack level. Getting the cell state of health information requires next-generation BMS. In addition, because a lot of factors could contribute to the low SOH at the end of life, such as temperature during operation, charging/discharging pattern and calendar degradation, the degradation mechanism could be different. Thus, just knowing the SOH is not enough to ensure the quality of the refurbished pack. To solve this challenge, engineers can mitigate the degradation by engineering the next-generation thermal management system. To fully understand the degradation inside the battery, computational methods including the first-principle method, physics-based model and machine learning based method should work together to identify the different degradation modes and quantify the level of degradation after severe operations. Lastly, more efficient battery characteristics tools, for instance, electrochemical impedance spectroscopy (EIS) should be used to ensure the quality of the battery pack.[40][41]

Second, it is costly and time-intensive to disassemble modules and cells. Following the last point, the first step is testing to determine the remaining SOH of the battery modules. This operation could vary for each retired system. Next, the module must be fully discharged. Then, the pack must be disassembled and reconfigured to meet the power and energy requirement of the second life application. It is important to note that qualified workers and specialized tools are required to dismantle the high weight and high voltage EV batteries. Besides the solutions discussed in the previous section, a refurbishing company can sell or reuse the discharged energy from the module to reduce the cost of this process. To accelerate the disassembly process, there have been several attempts to incorporate robots in this process. In this case, robots can handle more dangerous task increasing the safety of the dismantling process.[40][42]
Third, battery technology is non-transparent and lacks standards. Because battery development is the core part of EV, it is difficult for the manufacturer to label the exact chemistry of cathode, anode and electrolytes on the pack. In addition, the capacity and the design of the cells and packs changes on a yearly basis. The refurbishing company needs to closely work with the manufacture to have a timely update on this information. On the other hand, government can set up labeling standard.[40]
Lastly, the refurbishing process adds cost to the used batteries. Since 2010, the battery costs have decreased by over 85% which is significantly faster than the prediction. Because of the added cost of refurbishing, the refurbished unit may be less attractive than the new batteries to the market.[40]
Nonetheless, there have been several successes on the second-life application as shown in the examples of storage projects using second-life EV batteries. They are used in less demanding stationary storage application as peak shaving or additional storage for renewable-based generating sources.[40]
Recycling[edit]

Although battery life span can be extended by enabling a second-life application, ultimately EV batteries need to be recycled. Recyclability is not currently an important design consideration for battery manufacturers, and in 2019 only 5% of electric vehicle batteries were recycled.[43] BEV technologies lack an established recycling framework in many countries, making the usage of BEV and other battery-operated electrical equipment a large energy expenditure, ultimately increasing CO2 emissions - especially in countries lacking renewable energy resources.[44] Currently, there are five types of recycling processes: Pyrometallurgical recovery, Physical materials separation, Hydrometallurgical metal reclamation, Direct recycling method and Biological metals reclamation. The most widely used processes are the first three processes listed, as shown in the examples of current lithium-ion battery recycling facilities. The last two methods are still on lab or pilot scale, however, they can potentially avoid the largest amount of emission from mining.
The pyrometallurgical process involves burning the battery materials with slag, limestone, sand and coke to produce a metal alloy using a high-temperature furnace. The resulted materials are a metallic alloy, slag and gases. The gases comprise molecules that are evaporated from the electrolyte and binder components. The metal alloy can be separated through hydrometallurgical processes into constituent materials. The slag which is a mixture of metals aluminum, manganese and lithium can either be reclaimed by hydrometallurgical processes or used in the cement industry. This process is very versatile and relatively safe. Because there is no pre-sorting needed, it can work with a wide variety of batteries. In addition, because the whole cell is burnt, the metal from the current collectors could help the smelting process and because of the exothermic reaction of burning electrolyte sand plastics the energy consumption can also be reduced. However, this process still requires relatively higher energy consumption and only a limited number of materials can be reclaimed. Physical materials separation recovered materials by mechanical crushing and exploiting physical properties of different components such as particle size, density, ferromagnetism and hydrophobicity. Copper, aluminum and steel casing can be recovered by sorting. The remaining materials, called "black mass", which is composed of nickel, cobalt, lithium and manganese, need a secondary treatment to recover. For the hydrometallurgical process, the cathode materials need to be crushed to remove the current collector. Then, the cathode materials are leached by aqueous solutions to extract the desired metals from cathode materials. Direct cathode recycling as the name suggested extracts the materials directly, yielding a cathode power that is ready to be used as new cathode pristine material. This process involves extracting the electrolyte using liquid or supercritical CO2. After the size of the recovered components is reduced, the cathode materials can be separated out. For the biological metals reclamation or bio-leaching, the process uses microorganisms to digest metal oxides selectively. Then, recyclers can reduce these oxides to produce metal nanoparticles. Although bio-leaching has been used successfully in the mining industry, this process is still nascent to the recycling industry and plenty of opportunities exists for further investigation.[38][40][42]
There have been many efforts around the world to promote recycling technologies development and deployment. In the US, the Department of Energy Vehicle Technologies Offices (VTO) set up two efforts targeting at innovation and practicability of recycling processes. ReCell Lithium Recycling RD center brings in three universities and three national labs together to develop innovative, efficient recycling technologies. Most notably, the direct cathode recycling method was developed by the ReCell center. On the other hand, VTO also set up the battery recycling prize to incentivize American entrepreneurs to find innovative solutions to solve current challenges.[45]
Environmental impact[edit]
Transition to electric vehicles is estimated to require 87 times more than 2015 of specific metals by 2060 that need to be mined initially, with recycling (see above) covering part of the demand in future.[46] According to IEA 2021 study, mineral supplies need to increase from 400 kilotonnes in 2020 to 11,800 kilotonnes in 2040 in order to cover the demand by EV. This increase creates a number of key challenges, from supply chain (as 60% of production is concentrated in China) to significant impact on climate[need quotation to verify] and environment as result of such a large increase in mining operations.[47] However 45% of oil demand in 2022 was for road transport, and batteries may reduce this to 20% by 2050,[48] which would save hundreds of times more raw material than that used to make the batteries.[49]
Battery cost[edit]

In 2010, scientists at the Technical University of Denmark paid US$10,000 for a certified EV battery with 25kWh capacity (i.e. US$400/kWh), with no rebates or surcharges.[52] Two out of 15 battery producers could supply the necessary technical documents about quality and fire safety.[53] In 2010 it was estimated that at most 10years would pass before the battery price would come down to one-third.[52]
According to a 2010 study, by the United States National Research Council, the cost of a lithium-ion battery pack was about US$1,700/kWh of usable energy, and considering that a PHEV-10 requires about 2.0kWh and a PHEV-40 about 8kWh, the manufacturer cost of the battery pack for a PHEV-10 is around US$3,000 and it goes up to US$14,000 for a PHEV-40.[54][55] The MIT Technology Review estimated the cost of automotive battery packs to be between US$225 to US$500 per kilowatt hour by 2020.[56] A 2013 study by the American Council for an Energy-Efficient Economy reported that battery costs came down from US$1,300/kWh in 2007 to US$500/kWh in 2012. The U.S. Department of Energy has set cost targets for its sponsored battery research of US$300/kWh in 2015 and US$125/kWh by 2022. Cost reductions through advances in battery technology and higher production volumes will allow plug-in electric vehicles to be more competitive with conventional internal combustion engine vehicles.[57] In 2016, the world had a Li-ion production capacity of 41.57GWh.[58]
The actual costs for cells are subject to much debate and speculation as most EV manufacturers refuse to discuss this topic in detail. However, in October 2015, car maker GM revealed at their annual Global Business Conference that they expected a price of US$145/kWh for Li-ion cells entering 2016, substantially lower than other analysts' cost estimates. GM also expects a cost of US$100/kWh by the end of 2021.[59]
According to a study published in February 2016 by Bloomberg New Energy Finance (BNEF), battery prices fell 65% since 2010, and 35% just in 2015, reaching US$350/kWh. The study concludes that battery costs are on a trajectory to make electric vehicles without government subsidies as affordable as internal combustion engine cars in most countries by 2022. BNEF projects that by 2040, long-range electric cars will cost less than US$22,000 expressed in 2016 dollars. BNEF expects electric car battery costs to be well below US$120/kWh by 2030, and to fall further thereafter as new chemistries become available.[60][61]
- Battery cost estimate comparison
| Battery type | Year | Cost (US$/kWh) |
|---|---|---|
| Li-ion | 2021 | 132[62] |
| Li-ion | 2016 | 130[63]-145[59] |
| Li-ion | 2014 | 200300[64] |
| Li-ion | 2012 | 500600[65] |
| Li-ion | 2012 | 400[66] |
| Li-ion | 2012 | 520650[67] |
| Li-ion | 2012 | 752[67] |
| Li-ion | 2012 | 689[67] |
| Li-ion | 2013 | 8001000[68] |
| Li-ion | 2010 | 750[69] |
| Nickelmetal hydride | 2004 | 750[70] |
| Nickelmetal hydride | 2013 | 500550[68] |
| Nickelmetal hydride | 350[71] | |
| Leadacid | 256.68 |
EV parity[edit]

Cost parity[edit]
Different costs are important. One issue is purchase price, the other issue is total cost of ownership. Total cost of ownership of electric cars is often less than petrol or diesel cars.[73] In 2024 Gartner predicted that by 2027, next-generation BEVs will, on average, be cheaper to produce than a comparable ICE.[74]
Range parity[edit]
Driving range parity means that the electric vehicle has the same range as an average all-combustion vehicle (500 kilometres or 310 miles), with batteries of specific energy greater than 1kWh/kg.[75] Higher range means that the electric vehicles would run more kilometers without recharge. Currently, electric vehicle sales are lower than expected due range anxiety[citation needed] - even with the same range as an average all-combustion vehicle, buyers must be assured that there are widely available and compatible charging stations for their vehicles.[76]
As of 2024[update] the range of electric ships and large planes is less than combustion engined ones. To electrify all shipping standardized multi-megawatt charging is needed.[77] But sometimes batteries can be swapped, for example for river shipping.[78] As of 2024[update] pure electric large plane ranges of over 1000km are not expected within a decade - meaning that for over half of scheduled flights range parity cannot be acheived.[79]
Specifics[edit]
Internal components[edit]


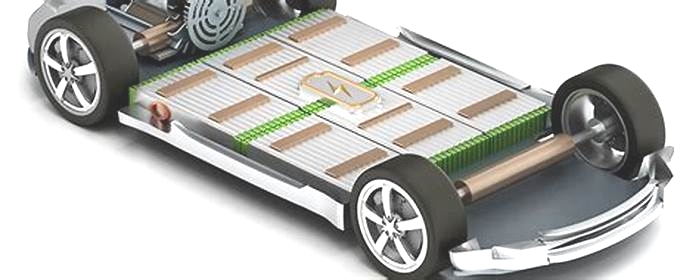
Battery pack designs for electric vehicles (EVs) are complex and vary widely by manufacturer and specific application. However, they all incorporate a combination of several simple mechanical and electrical component systems which perform the basic required functions of the pack.[citation needed]
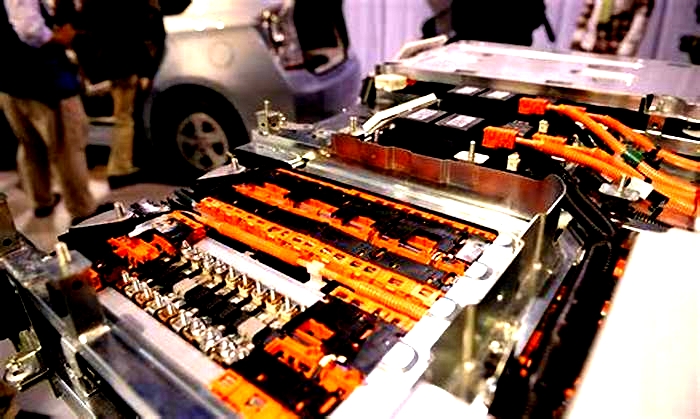
The actual battery cells can have different chemistry, physical shapes, and sizes as preferred by various pack manufacturers. Battery packs will always incorporate many discrete cells connected in series and parallel to achieve the total voltage and current requirements of the pack. Battery packs for all electric drive EVs can contain several hundred individual cells. Each cell has a nominal voltage of 3-4 volts, depending on its chemical composition.[citation needed]
To assist in manufacturing and assembly, the large stack of cells is typically grouped into smaller stacks called modules. Several of these modules are placed into a single pack. Within each module the cells are welded together to complete the electrical path for current flow. Modules can also incorporate cooling mechanisms, temperature monitors, and other devices. Modules must remain within a specific temperature range for optimal performance.[80] In most cases, modules also allow for monitoring the voltage produced by each battery cell in the stack by using a battery management system (BMS).[81]
The battery cell stack has a main fuse which limits the current of the pack under a short circuit. A "service plug" or "service disconnect" can be removed to split the battery stack into two electrically isolated halves. With the service plug removed, the exposed main terminals of the battery present no high potential electrical danger to service technicians.[81][82]
The battery pack also contains relays, or contactors, which control the distribution of the battery pack's electrical power to the output terminals. In most cases there will be a minimum of two main relays which connect the battery cell stack to the main positive and negative output terminals of the pack, which then supply high current to the electrical drive motor. Some pack designs include alternate current paths for pre-charging the drive system through a pre-charge resistor or for powering an auxiliary bus which will also have their own associated control relays. For safety reasons these relays are all normally open.[81][82]
The battery pack also contains a variety of temperature, voltage, and current sensors. Collection of data from the pack sensors and activation of the pack relays are accomplished by the pack's battery monitoring unit (BMU) or BMS. The BMS is also responsible for communications with the vehicle outside the battery pack.[81]
Recharging[edit]
Batteries in BEVs must be periodically recharged. BEVs most commonly charge from the power grid (at home or using a street or shop recharging point), which is in turn generated from a variety of domestic resources, such as coal, hydroelectricity, nuclear, natural gas, and others. Home or grid power, such as photovoltaic solar cell panels, wind, or microhydro may also be used and are promoted because of concerns regarding global warming.
With suitable power supplies, good battery lifespan is usually achieved at charging rates not exceeding half of the capacity of the battery per hour ("0.5C"),[83] thereby taking two or more hours for a full charge, but faster charging is available even for large capacity batteries.[84]
Charging time at home is limited by the capacity of the household electrical outlet, unless specialized electrical wiring work is done. In the US, Canada, Japan, and other countries with 120V electricity, a normal household outlet delivers 1.5 kilowatts. In other countries with 230V electricity between 7 and 14 kilowatts can be delivered (230V single phase and 400V three-phase, respectively). In Europe, a 400V (three-phase 230V) grid connection is increasingly popular since newer houses don't have natural gas connection due to the European Union's safety regulations.[citation needed]
Recharging time[edit]
Electric cars like Tesla Model S, Renault Zoe, BMW i3, etc., can recharge their batteries to 80 percent at quick charging stations within 30 minutes.[85][86][87][88] For example, a Tesla Model 3 Long Range charging on a 250kW Tesla Version 3 Supercharger went from 2% state of charge with 6 miles (9.7km) of range to 80% state of charge with 240 miles (390km) of range in 27 minutes, which equates to 520 miles (840km) per hour.[89]
Connectors[edit]
The charging power can be connected to the car in two ways. The first is a direct electrical connection known as conductive coupling. This might be as simple as a mains lead into a weatherproof socket through special high capacity cables with connectors to protect the user from high voltages. The modern standard for plug-in vehicle charging is the SAE1772 conductive connector (IEC62196 Type1) in the US. The ACEA has chosen the VDE-AR-E 2623-2-2 (IEC62196 Type2) for deployment in Europe, which, without a latch, means unnecessary extra power requirements for the locking mechanism.[citation needed]
The second approach is known as inductive charging. A special 'paddle' is inserted into a slot on the car. The paddle is one winding of a transformer, while the other is built into the car. When the paddle is inserted it completes a magnetic circuit which provides power to the battery pack. In one inductive charging system,[90] one winding is attached to the underside of the car, and the other stays on the floor of the garage. The advantage of the inductive approach is that there is no possibility of electrocution as there are no exposed conductors, although interlocks, special connectors and ground fault detectors can make conductive coupling nearly as safe. Inductive charging can also reduce vehicle weight, by moving more charging componentry offboard.[91] An inductive charging advocate from Toyota contended in 1998, that overall cost differences were minimal, while a conductive charging advocate from Ford contended that conductive charging was more cost efficient.[91]
Recharging spots[edit]
As of April2020[update], there are 93,439 locations and 178,381 EV charging stations worldwide.[92]
Though there are a lot of charging stations worldwide, and the number is only growing, an issue with this is that an EV driver may find themselves at a remote charging station with another vehicle plugged in to the only charger or they may find another vehicle parked in the only EV spot. Currently, no laws prohibit unplugging another person's vehicle, it is simply ruled by etiquette.[76]
Travel range before recharging[edit]
The range of a BEV depends on the number and type of batteries used. The weight and type of vehicle as well as terrain, weather, and the performance of the driver also have an impact, just as they do on the mileage of traditional vehicles. Electric vehicle conversion performance depends on a number of factors including the battery chemistry:
- leadacid batteries are the most available and inexpensive. Such conversions generally have a range of 3080km (1950mi). Production EVs with leadacid batteries are capable of up to 130km (81mi) per charge.
- NiMH batteries have higher specific energy than leadacid; prototype EVs deliver up to 200km (120mi) of range.
- New lithium-ion battery-equipped EVs provide 320540km (200340mi) of range per charge.[93][94] Lithium is also less expensive than nickel.[95]
- nickelzinc batteries are cheaper and lighter than nickelcadmium batteries. They are also cheaper than (but not as light as) lithium-ion batteries.[96]
The internal resistance of some batteries may be significantly increased at low temperature[97] which can cause noticeable reduction in the range of the vehicle and on the lifetime of the battery.
Finding the economic balance of range versus performance, battery capacity versus weight, and battery type versus cost challenges every EV manufacturer.
With an AC system or advanced DC system, regenerative braking can extend range by up to 50% under extreme traffic conditions without complete stopping. Otherwise, the range is extended by about 10 to 15% in city driving, and only negligibly in highway driving, depending upon terrain.[citation needed]
BEVs (including buses and trucks) can also use genset trailers and pusher trailers in order to extend their range when desired without the additional weight during normal short range use. Discharged basket trailers can be replaced by recharged ones en route. If rented then maintenance costs can be deferred to the agency.
Some BEVs can become hybrid vehicles depending on the trailer and car types of energy and powertrain.
Trailers[edit]
Auxiliary battery capacity carried in trailers can increase the overall vehicle range, but also increases the loss of power arising from aerodynamic drag, increases weight transfer effects and reduces traction capacity.
Swapping and removing[edit]
An alternative to recharging is to exchange drained or nearly drained batteries (or battery range extender modules) with fully charged batteries. This is called battery swapping and is done in exchange stations.[98]
Features of swap stations include:[99]
- The consumer is no longer concerned with battery capital cost, life cycle, technology, maintenance, or warranty issues;
- Swapping is far faster than charging: battery swap equipment built by the firm Better Place has demonstrated automated swaps in less than 60seconds;[100]
- Swap stations increase the feasibility of distributed energy storage via the electric grid;
Concerns about swap stations include:
- Potential for fraud (battery quality can only be measured over a full discharge cycle; battery lifetime can only be measured over repeated discharge cycles; those in the swap transaction cannot know if they are getting a worn or reduced effectiveness battery; battery quality degrades slowly over time, so worn batteries will be gradually forced into the system)
- Manufacturers' unwillingness to standardize battery access / implementation details[101]
- Safety concerns[101]
Vehicle-to-grid[edit]
Smart grid allows BEVs to provide power to the grid at any time, especially:
- During peak load periods (When the selling price of electricity can be very high. Vehicles can then be recharged during off-peak hours at cheaper rates which helps absorb excess night time generation. The vehicles serve as a distributed battery storage system to buffer power.)
- During blackouts, as backup power sources.
The safety issues of battery electric vehicles are largely dealt with by the international standard ISO 6469. This standard is divided into three parts:
- On-board electrical energy storage, i.e. the battery
- Functional safety means and protection against failures
- Protection of persons against electrical hazards.
Firefighters and rescue personnel receive special training to deal with the higher voltages and chemicals encountered in electric and hybrid electric vehicle accidents. While BEV accidents may present unusual problems, such as fires and fumes resulting from rapid battery discharge, many experts agree that BEV batteries are safe in commercially available vehicles and in rear-end collisions, and are safer than gasoline-propelled cars with rear gasoline tanks.[102]
Usually, battery performance testing includes the determination of:
Performance testing simulates the drive cycles for the drive trains of Battery Electric Vehicles (BEV), Hybrid Electric Vehicles (HEV) and Plug in Hybrid Electric Vehicles (PHEV) as per the required specifications of car manufacturers (OEMs). During these drive cycles, controlled cooling of the battery can be performed, simulating the thermal conditions in the car.
In addition, climatic chambers control environmental conditions during testing and allow simulation of the full automotive temperature range and climatic conditions.[citation needed]
Patents[edit]
Patents may be used to suppress development or deployment of battery technology. For example, patents relevant to the use of Nickel metal hydride cells in cars were held by an offshoot of Chevron Corporation, a petroleum company, who maintained veto power over any sale or licensing of NiMH technology.[103][104]
Research, development and innovation[edit]
As of December 2019, billions of euro in research are planned to be invested around the world for improving batteries.[105][106]
Researchers have come up with some design considerations for contactless BEV chargers. Inductively coupled power transfer (ICPT) systems are made to transfer power efficiently from a primary source (charging station) to one or more secondary sources (BEVs) in a contactless way via magnetic coupling.[107]
Europe has plans for heavy investment in electric vehicle battery development and production, and Indonesia also aims to produce electric vehicle batteries in 2023, inviting Chinese battery firm GEM and Contemporary Amperex Technology Ltd to invest in Indonesia.[108][109][110][111][112][113][114][115]
Ultracapacitors[edit]
Electric double-layer capacitors (or "ultracapacitors") are used in some electric vehicles, such as AFS Trinity's concept prototype, to store rapidly available energy with their high specific power, in order to keep batteries within safe resistive heating limits and extend battery life.[116][117]
Since commercially available ultracapacitors have a low specific energy, no production electric cars use ultracapacitors exclusively.
In January 2020, Elon Musk, CEO of Tesla, stated that the advancements in Li-ion battery technology have made ultra-capacitors unnecessary for electric vehicles.[118]
Promotion in the United States[edit]
On 2 May 2022, President Biden announced the administration will begin a $3.16 billion plan to boost domestic manufacturing and recycling of batteries, in a larger effort to shift the country away from gas-powered cars to electric vehicles. The goal of the Biden administration is to have half of U.S. automobile production electric by 2030.[119]
The Inflation Reduction Act, passed on 16 August 2022, aimed to incentivize clean energy manufacturing with a $7,500 consumer tax credit for EVs with US-built batteries, and subsidies for EV plants. By October 2022, billions of dollars of investment had been announced for over two dozen US battery plants, leading some commentators to nickname the Midwest as the "Battery Belt".[120][121]
See also[edit]
Examples[edit]
Related[edit]
References[edit]
- ^ "Axeon Receives Order for 50 Zebra Packs for Modec Electric Vehicle; Li-Ion Under Testing". Green Car Congress. 24 November 2016. Retrieved 15 December 2019.
- ^ "Battery prices are falling, which is good news for EVs". Marketplace. 3 December 2019. Retrieved 25 April 2020.
- ^ "EV Database". EV Database. Retrieved 25 April 2020.
- ^ "Electric vs. Gas Cars: Is It Cheaper to Drive an EV?". www.nrdc.org. 21 March 2024. Retrieved 12 April 2024.
These savings are largely based on the fact that current EVs are 2.6 to 4.8 times more efficient at traveling a mile compared to a gasoline internal combustion engine, according to real world data collected by the U.S. Department of Energy (DOE).
- ^ Godshall, N.A.; Raistrick, I.D.; Huggins, R.A. (1980). "Thermodynamic investigations of ternary lithium-transition metal-oxygen cathode materials". Materials Research Bulletin. 15 (5): 561. doi:10.1016/0025-5408(80)90135-X.
- ^ Godshall, Ned A. (18 May 1980) Electrochemical and Thermodynamic Investigation of Ternary Lithium-Transition Metal-Oxygen Cathode Materials for Lithium Batteries. Ph.D. Dissertation, Stanford University
- ^ "USPTO search for inventions by "Goodenough, John"". Patft.uspto.gov. Archived from the original on 25 February 2021. Retrieved 8 October 2011.
- ^ Mizushima, K.; Jones, P. C.; Wiseman, P. J.; Goodenough, J. B. (1980). "Li xCoO 2(0<x<-1): A new cathode material for batteries of high energy density". Materials Research Bulletin. 15 (6): 783789. doi:10.1016/0025-5408(80)90012-4. S2CID97799722.
- ^ Jalkanen, K.; Karrpinen, K.; Skogstrom, L.; Laurila, T.; Nisula, M.; Vuorilehto, K. (2015). "Cycle aging of commercial NMC/graphite pouch cells at different temperatures". Applied Energy. 154: 160172. Bibcode:2015ApEn..154..160J. doi:10.1016/j.apenergy.2015.04.110.
- ^ "Lithium-Ion Batteries Hazard and Use Assessment" (PDF). Retrieved 7 September 2013.[permanent dead link]
- ^ "A123 Inks Deal to Develop Battery Cells for GM Electric Car". 10 August 2007. Retrieved 10 December 2016.
- ^ "Li-Ion Rechargeable Batteries Made Safer". Nikkei Electronics Asia. February 2008. Archived from the original on 12 September 2011.
- ^ Kurzweil, Peter (1 January 2015), Moseley, Patrick T.; Garche, Jrgen (eds.), "Chapter 16 - Lithium Battery Energy Storage: State of the Art Including LithiumAir and LithiumSulfur Systems", Electrochemical Energy Storage for Renewable Sources and Grid Balancing, Amsterdam: Elsevier, pp.269307, ISBN978-0-444-62616-5, retrieved 15 December 2023
- ^ "Nanowire battery can hold 10 times the charge of existing lithium-ion battery". 9 January 2008. Archived from the original on 7 January 2010. Retrieved 10 December 2016.
- ^ Cui, Yi. "Inorganic Nanowires as Advanced Energy Conversion and Storage Materials" (PDF). US: Stanford University. Archived from the original (PDF) on 13 May 2016. Retrieved 31 March 2019.
- ^ Jaques, Robert (14 April 2008). "Nanotech promises lithium ion battery boost". vnunet.com. Archived from the original on 8 April 2009. Retrieved 3 October 2013.
- ^ "Using nanotechnology to improve Li-ion battery performance". Retrieved 10 December 2016.
- ^ Zhang, Wei-Ming; Hu, Jin-Song; Guo, Yu-Guo; Zheng, Shu-Fa; Zhong, Liang-Shu; Song, Wei-Guo; Wan, Li-Jun (2008). "Tin-Nanoparticles Encapsulated in Elastic Hollow Carbon Spheres for High-Performance Anode Material in Lithium-Ion Batteries". Advanced Materials. 20 (6): 11601165. Bibcode:2008AdM....20.1160Z. doi:10.1002/adma.200701364. S2CID95337256.
- ^ "Argonne's lithium-ion battery technology to be commercialized by Japan's Toda Kogyo". Retrieved 10 December 2016.
- ^ Johnson, Christopher S. (2007). "Journal of Power Sources: Development and utility of manganese oxides as cathodes in lithium batteries". Journal of Power Sources. 165 (2): 559565. doi:10.1016/j.jpowsour.2006.10.040.
- ^ "Hybrid Develops New "Superlattice Structure" Lithium Battery Capable of Increasing Drive Ranges in Excess of 200 Miles". Hybrid Technologies. US. 24 February 2008. Archived from the original on 2 March 2008.
- ^ "New Data Shows Heat & Fast-Charging Responsible For More Battery Degradation Than Age Or Mileage". CleanTechnica. 16 December 2019.
- ^ a b "Lithium-ion Battery (LFP and NMC)". www.pnnl.gov. Retrieved 13 April 2024.
- ^ "Why are LFP Cells so Attractive?". springerprofessional.de (in German). 12 April 2024. Retrieved 13 April 2024.
- ^ "Nickel Metal Hydride NiMH Batteries". mpoweruk.com. Retrieved 26 April 2020.
- ^ "GM, Chevron and CARB killed the sole NiMH EV once, will do so again Plug-in Electric cars and solar power reduce dependence on foreign oil by living oil-free, we review the options". Retrieved 26 April 2020.
- ^ a b Barre, Harold (1997). Managing 12Volts: How To Upgrade, Operate, and Troubleshoot 12Volt Electrical Systenms. Summer Breeze Publishing. pp.6365. ISBN978-0-9647386-1-4.(discussing damage caused by sulfation due to discharge below 50%)
- ^ "Modec electric truck - DIY Electric Car Forums". diyelectriccar.com. Retrieved 26 April 2020.
- ^ "Full Electric". Sustainable Ships. Retrieved 15 April 2024.
- ^ a b Global Supply Chains of EV Batteries. International Energy Agency. 2022.
- ^ Mills, Ryan (8 March 2023). "EV Batteries 101: Supply Chains". Rocky Mountain Institute. Retrieved 17 April 2023.
- ^ Zeng, Anqi; Chen, Wu; Rasmussen, Kasper Dalgas; Zhu, Xuehong; Lundhaug, Maren; Mller, Daniel B.; Tan, Juan; Keiding, Jakob K.; Liu, Litao; Dai, Tao; Wang, Anjian; Liu, Gang (15 March 2022). "Battery technology and recycling alone will not save the electric mobility transition from future cobalt shortages". Nature Communications. 13 (1): 1341. doi:10.1038/s41467-022-29022-z. ISSN2041-1723. PMC8924274. PMID35292628.
- ^ Ziegler, Bart (12 November 2022). "Electric Vehicles Require Lots of Scarce Parts. Is the Supply Chain Up to It?". Wall Street Journal. Retrieved 26 April 2023.
- ^ "How electric vehicles are accelerating the end of the oil age".
- ^ a b "Electric vehicles, second life batteries, and their effect on the power sector | McKinsey". mckinsey.com. Retrieved 10 May 2021.
- ^ Zhao, Yanyan; Pohl, Oliver; Bhatt, Anand I.; Collis, Gavin E.; Mahon, Peter J.; Rther, Thomas; Hollenkamp, Anthony F. (9 March 2021). "A Review on Battery Market Trends, Second-Life Reuse, and Recycling". Sustainable Chemistry. 2 (1): 167205. doi:10.3390/suschem2010011. ISSN2673-4079.
- ^ Xu, Chengjian; Dai, Qiang; Gaines, Linda; Hu, Mingming; Tukker, Arnold; Steubing, Bernhard (December 2020). "Future material demand for automotive lithium-based batteries". Communications Materials. 1 (1): 99. Bibcode:2020CoMat...1...99X. doi:10.1038/s43246-020-00095-x. hdl:1887/138961. ISSN2662-4443.
- ^ a b c d Ciez, Rebecca E.; Whitacre, J. F. (February 2019). "Examining different recycling processes for lithium-ion batteries". Nature Sustainability. 2 (2): 148156. Bibcode:2019NatSu...2..148C. doi:10.1038/s41893-019-0222-5. ISSN2398-9629. S2CID188116440.
- ^ Schmuch, Richard; Wagner, Ralf; Hrpel, Gerhard; Placke, Tobias; Winter, Martin (April 2018). "Performance and cost of materials for lithium-based rechargeable automotive batteries". Nature Energy. 3 (4): 267278. Bibcode:2018NatEn...3..267S. doi:10.1038/s41560-018-0107-2. ISSN2058-7546. S2CID139370819.
- ^ a b c d e f g h Global EV Outlook 2020. 18 June 2020. doi:10.1787/d394399e-en. ISBN9789264616226. S2CID242162623.
- ^ Assessment of Technologies for Improving Light-Duty Vehicle Fuel Economy"2025-2035. The National Academies Press. 2021. doi:10.17226/26092. ISBN978-0-309-37122-3. S2CID234202631.
- ^ a b Harper, Gavin; Sommerville, Roberto; Kendrick, Emma; Driscoll, Laura; Slater, Peter; Stolkin, Rustam; Walton, Allan; Christensen, Paul; Heidrich, Oliver; Lambert, Simon; Abbott, Andrew (6 November 2019). "Recycling lithium-ion batteries from electric vehicles". Nature. 575 (7781): 7586. Bibcode:2019Natur.575...75H. doi:10.1038/s41586-019-1682-5. ISSN0028-0836. PMID31695206.
- ^ Jacoby, Mitch (14 July 2019). "It's time to get serious about recycling lithium-ion batteries". Chemical & Engineering News.
- ^ Manzetti, Sergio; Mariasiu, Florin (1 November 2015). "Electric vehicle battery technologies: From present state to future systems". Renewable and Sustainable Energy Reviews. 51: 10041012. doi:10.1016/j.rser.2015.07.010. ISSN1364-0321.
- ^ Author, Not Given (1 April 2019). "FY2018 Batteries Annual Progress Report". doi:10.2172/1525362. OSTI1525362. S2CID243075830. ;
- ^ Mnberger, Andr; Stenqvist, Bjrn (1 August 2018). "Global metal flows in the renewable energy transition: Exploring the effects of substitutes, technological mix and development". Energy Policy. 119: 226241. Bibcode:2018EnPol.119..226M. doi:10.1016/j.enpol.2018.04.056. ISSN0301-4215. S2CID52227957.
- ^ "The Role of Critical Minerals in Clean Energy Transitions Analysis". IEA. 5 May 2021. Archived from the original on 17 June 2021. Retrieved 16 June 2021. Alt URL[permanent dead link]
- ^ "How EVs Will Drive Peak Oil This Decade, in Five Charts". BloombergNEF. 22 June 2023. Retrieved 29 March 2024.
- ^ "Batteries vs oil: A comparison of raw material needs". Transport & Environment. 1 March 2021. Retrieved 29 March 2024.
- ^ Ziegler, Micah S.; Trancik, Jessika E. (2021). "Re-examining rates of lithium-ion battery technology improvement and cost decline". Energy & Environmental Science. 14 (4): 16351651. doi:10.1039/D0EE02681F. hdl:1721.1/132660. ISSN1754-5692. S2CID220830992.
- ^ "The price of batteries has declined by 97% in the last three decades". Our World in Data. Retrieved 26 April 2022.
- ^ a b Bredsdorff, Magnus (22 June 2010). "Et batteri til en elbil koster 60.000 kroner" [Electrical Vehicle battery costs $10,000]. Ingeniren (in Danish). Archived from the original on 25 June 2010. Retrieved 30 January 2017.
- ^ Bredsdorff, Magnus (22 June 2010). "EV batteries still prototypes". Ingeniren (in Danish). Denmark. Archived from the original on 25 June 2010. Retrieved 22 June 2010.
- ^ National Research Council (2010). Transitions to Alternative Transportation Technologies--Plug-in Hybrid Electric Vehicles. The National Academies Press. doi:10.17226/12826. ISBN978-0-309-14850-4. Archived from the original on 7 June 2011. Retrieved 3 March 2010.
- ^ Jad Mouawad and Kate Galbraith (14 December 2009). "Study Says Big Impact of the Plug-In Hybrid Will Be Decades Away". The New York Times. Retrieved 4 March 2010.
- ^ Tommy McCall (25 June 2011). "THE PRICE OF BATTERIES" (PDF). MIT Technology Review. Retrieved 5 May 2017.
- ^ Siddiq Khan and Martin Kushler (June 2013). "Plug-in Electric Vehicles: Challenges and Opportunities" (PDF). American Council for an Energy-Efficient Economy. Retrieved 9 July 2013. ACEEE Report Number T133.
- ^ Gibbs, Nick (2 January 2017). "Automakers hunt for battery cell capacity to deliver on bullish EV targets". Automotive News. Archived from the original on 9 January 2017. Retrieved 9 January 2017.
- ^ a b Cobb, Jeff (2 October 2015). "Chevy Bolt Production Confirmed For 2016". Hybrid cars. Retrieved 14 December 2015.
- ^ Randall, Tom (25 February 2016). "Here's How Electric Cars Will Cause the Next Oil Crisis". Bloomberg News. Retrieved 26 February 2016. See embedded video.
- ^ Bloomberg New Energy Finance (25 February 2016). "Here's How Electric Cars Will Cause the Next Oil Crisis" (Press release). London and New York: PR Newswire. Retrieved 26 February 2016.
- ^ Firth, James (30 November 2021). "Battery Price Declines Slow Down in Latest Pricing Survey". Bloomberg Green. Bloomberg News. Retrieved 1 December 2021.
- ^ Dallkken, Per Erlien (23 December 2016). "Her produseres elbilen og bensinbilen p samme linje" [Electric car and petrol truck produced on the same line]. Teknisk Ukeblad (in Norwegian). Norway. Retrieved 16 August 2018.
- ^ "Tesla to Miss 2020 Delivery Target by 40%, Analyst Forecasts". greentechmedia.com. 17 December 2014. Retrieved 28 January 2015.
Tesla's current batteries cost $200-$300 per kilowatt hour.
- ^ "Battery technology charges ahead | McKinsey & Company". mckinsey.com. Archived from the original on 22 January 2014. Retrieved 1 February 2014.
- ^ "Lithium-ion battery costs will still be about $400/kWh by 2020". green.autoblog.com. Retrieved 1 February 2014.
- ^ a b c "McKinsey: Lithium Ion Battery Prices to Reach $200/kWh by 2020 | PluginCars.com". plugincars.com. Retrieved 1 February 2014.
- ^ a b "Tesla Debacle Highlights Need For New EV Battery Technology - Forbes". forbes.com. Retrieved 1 February 2014.
- ^ "WSJ: Nissan Leaf profitable by year three; battery cost closer to $18,000". green.autoblog.com. Retrieved 1 February 2014.
- ^ Anderman, Menahem (2003). "Brief Assessment of Improvements in EV BatteryTechnology since the BTAP June 2000 Report" (PDF). California Air Resources Board. Archived from the original (PDF) on 4 March 2016. Retrieved 16 August 2018.
- ^ "GM, Chevron and CARB killed the NiMH EV once, will do so again". ev1.org. Retrieved 1 February 2014.
- ^ a b "Race to Net Zero: The Pressures of the Battery Boom in Five Charts". 21 July 2022. Archived from the original on 7 September 2023.
- ^ "How much do electric vehicles (EVs) cost?". www.fleetnews.co.uk. Retrieved 15 April 2024.
- ^ "Gartner Outlines a New Phase for Electric Vehicles".
- ^ "Google Answers: Driving range for cars". Retrieved 1 February 2014.
- ^ a b Bonges, Henry A.; Lusk, Anne C. (1 January 2016). "Addressing electric vehicle (EV) sales and range anxiety through parking layout, policy and regulation". Transportation Research Part A: Policy and Practice. 83: 6373. doi:10.1016/j.tra.2015.09.011. ISSN0965-8564.
- ^ "Fast charging for battery-powered ships: Horizon Europe guarantee". www.ukri.org. 19 March 2024. Retrieved 15 April 2024.
- ^ "Largest Electric, Battery-Powered Containerships Commissioned in China". The Maritime Executive. Retrieved 15 April 2024.
- ^ "90-seat Elysian airliner: 800-1,000-km range on batteries alone". New Atlas. 12 January 2024. Retrieved 15 April 2024.
- ^ Duan, X.; Naterer, G. F. (1 November 2010). "Heat transfer in phase change materials for thermal management of electric vehicle battery modules". International Journal of Heat and Mass Transfer. 53 (23): 51765182. doi:10.1016/j.ijheatmasstransfer.2010.07.044. ISSN0017-9310.
- ^ a b c d "PHEV, HEV, and EV Battery Pack Testing in a Manufacturing Environment | DMC, Inc". dmcinfo.com.
- ^ a b "Leader of Battery Safety & Battery Regulation Programs - PBRA" (PDF). Archived from the original on 7 October 2011. Retrieved 7 September 2020.
- ^ Coren, Michael J. (15 December 2019). "Fast charging is not a friend of electric car batteries". Quartz. Retrieved 26 April 2020.
- ^ "How Long Does It Take to Charge an Electric Car?". J.D. Power. Retrieved 26 April 2020.
- ^ "Neue Stromtankstelle: Elektroautos laden in 20 Minuten". golem.de (in German). 15 September 2011.
- ^ Lbbehsen, Hanne (24 October 2013). "Elektroauto: Tesla errichtet Gratis-Schnellladestationen" [Electric car: Tesla builds free fast charging stations]. ZEIT ONLINE (in German). German. Retrieved 15 December 2019.
- ^ Die Akkus im Renault Zoe knnen in der schnellsten von vier Ladegeschwindigkeiten in 30 Minuten bis zu 80 Prozent aufgeladen werden, bild.de
- ^ Mit einem Schnellladegert lsst sich der Akku des i3 in nur 30 Minuten zu 80 Prozent aufladen, golem.de
- ^ "Tesla Model 3 V3 Supercharging Times: 2% To 100% State of Charge (Video)". CleanTechnica. 18 November 2019. Retrieved 26 April 2020.
- ^ "Site homepage". Retrieved 10 December 2016 via scitation.aip.org.
- ^ a b "Car Companies' Head-on Competition In Electric Vehicle Charging." (Website). The Auto Channel, 1998-11-24. Retrieved on 2007-08-21.
- ^ "Open Charge Map - Statistics". openchargemap.org. Retrieved 26 April 2020.
- ^ Mitchell, T (2003). "AC Propulsion Debuts tzero with LiIon Battery" (PDF) (Press release). AC Propulsion. Archived from the original (PDF) on 7 October 2003. Retrieved 5 July 2006.
- ^ https://www.edmunds.com/car-news/electric-car-range-and-consumption-epa-vs-edmunds.html
- ^ Gergely, Andras (21 June 2007). "Lithium batteries power hybrid cars of future: Saft". Reuters. US. Retrieved 22 June 2007.
- ^ Gunther, Marc (13 April 2009). "Warren Buffett takes charge". CNN. US. Retrieved 11 February 2017.
- ^ "US NREL: Electric Vehicle Battery Thermal Issues and Thermal Management" (PDF).
- ^ "Electric cars wait in the wings". Manawatu Standard. 17 September 2008. Retrieved 29 September 2011.
- ^ "Volkswagen Says 'No' to Battery Swapping, 'Yes' to Electrics in U.S.: Greentech Media". greentechmedia.com. 17 September 2009. Retrieved 1 February 2014.
- ^ "What's Hot: Car News, Photos, Videos & Road Tests | Edmunds.com". blogs.edmunds.com. Archived from the original on 7 July 2012. Retrieved 1 February 2014.
- ^ a b "Battery swap model ?won?t work? | carsguide.com.au". carsguide.com.au. Retrieved 3 March 2014.
- ^ Walford, Lynn (18 July 2014). "Are EV batteries safe? Electric car batteries can be safer than gas cars". auto connected car. Retrieved 22 July 2014.
- ^ "ECD Ovonics Amended General Statement of Beneficial Ownership". 2 December 2004. Archived from the original on 29 July 2009. Retrieved 8 October 2009.
- ^ "ECD Ovonics 10-Q Quarterly Report for the period ending March 31, 2008". 31 March 2008. Archived from the original on 28 July 2009. Retrieved 8 October 2009.
- ^ "EU approves 3.2 billion euro state aid for battery research". Reuters. 9 December 2019. Retrieved 10 December 2019.
- ^ "StackPath". tdworld.com. Retrieved 10 December 2019.
- ^ Wang, Chwei-Sen; Stielau, O.H.; Covic, G.A. (October 2005). "Design considerations for a contactless electric vehicle battery charger". IEEE Transactions on Industrial Electronics. 52 (5): 13081314. doi:10.1109/TIE.2005.855672. hdl:2292/243. ISSN1557-9948. S2CID13046022.
- ^ "Indonesia to produce EV batteries by 2022 - report". 19 December 2019.
- ^ "Factbox: Plans for electric vehicle battery production in Europe". Reuters. 9 November 2018 via www.reuters.com.
- ^ "European battery production to receive financial boost | DW | 02.05.2019". DW.COM. Archived from the original on 16 December 2019. Retrieved 16 December 2019.
- ^ "France and Germany commit to European electric battery industry". Reuters. 2 May 2019 via www.reuters.com.
- ^ "Europe aims to take its place on the global EV battery production stage". 28 March 2019.
- ^ "CATL Plans Massive Increase In European Battery Production". CleanTechnica. 27 June 2019.
- ^ "The 2040 outlook for EV battery manufacturing | McKinsey". mckinsey.com.
- ^ "EU aims to become powerhouse of battery production | Platts Insight". blogs.platts.com.
- ^ Wald, Matthew L. (13 January 2008). "Closing the Power Gap Between a Hybrid's Supply and Demand". The New York Times. Retrieved 1 May 2010.
- ^ "AFS TRINITY UNVEILS 150 MPG EXTREME HYBRID (XH) SUV" (PDF) (Press release). Archived from the original (PDF) on 29 February 2012. Retrieved 9 November 2009.
- ^ Lambert, Fred (21 January 2020). "Elon Musk: Tesla acquisition of Maxwell is going to have a very big impact on batteries". Electrek. Retrieved 26 April 2020.
- ^ Natter, Ari; Leonard, Jenny (2 May 2022). "Biden's Team Puts Up Over $3 Billion to Boost U.S. Battery Output". Bloomberg News. Retrieved 2 May 2022.
- ^ Weisbrod, Katelyn (27 October 2022). "The EV Battery Boom Is Here, With Manufacturers Investing Billions in Midwest Factories". Inside Climate News. Retrieved 29 October 2022.
- ^ Lewis, Michelle (13 October 2022). "Here's where the new US EV 'Battery Belt' is forming and why". Electrek. Retrieved 29 October 2022.